Two 'promising' Covid-19 treatments give Seattle-area patients and doctors hope
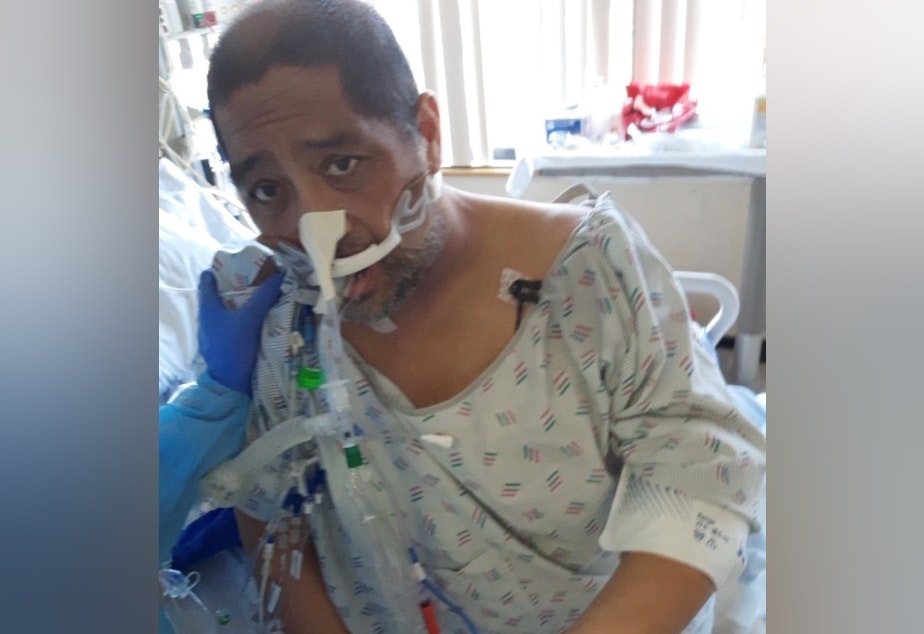
Very few medical professionals in the United States had seen a patient with Covid-19 when Raymond Sismaet showed up at his doctor’s office at the end of February complaining about a bad cough. On a return visit two days later he was sent by ambulance to the emergency room.
“And from then it got real hazy for me. I don’t remember being intubated and put on a ventilator,” Sismaet said.
He stayed on that ventilator for 18 days at the UW Medical Center. But Sismaet survived, in part due to the treatment he received while in the hospital.
It’s going to be a long, difficult wait for a conornavirus vaccine. But in the meantime, doctors in the Seattle area have already learned a lot about how to care for people with this disease, and many new treatments are being tried. This story takes a closer look at two remedies that may help.
Remdesivir
Because he was in isolation, Raymond’s spouse Jill Sismaet couldn’t see him in person, and she had to make a tough medical decision on her own: Whether to take a chance on an experimental drug called Remdesivir, which almost nobody had heard of at that time.
Sponsored
Watching her husband from outside the room, Sismaet thought he looked like he “needed a boost.” So she told the doctors to go for it.
Dr. Helen Chu, at the UW Medical Cente, said Remdisivir is an “antiviral drug” that got its start in the fight against Ebola. Today it’s being studied in a number of clinical trials, including an NIH trial, which includes some patients at the UW Medical Center.
The drug is supposed to stop the spread of the virus after you’ve been infected. It works by tricking the coronavirus into not making more of the coronavirus in your body.
And based on preliminary data from this randomized controlled trial the drug shows some promise. Hospitalized patients improved around 30% faster than those given a placebo. But there was no statistical improvement in mortality.
Raymond Sismaet got the drug just before the trial started, so he knows it was the real thing and not a placebo. He only got part of what was expected to be a 10 day intravenous treatment because his liver enzymes started to spike. That could be a sign of liver damage, which is a possible side effect from this drug.
Sponsored
But Jill Sismaet met does not regret the decision.
“If a person’s offered it, I personally would take it,” she said.
There’s no way of knowing for sure if the drug helped in Sismaet’s case. But the FDA has given emergency use authorization for the drug to treat Covid-19, and the World Health Organization is asking the manufacturer to make it more available to the rest of the world.
Antibodies
That brings us to the second promising treatment in this story, which also starts with a person who had the virus, Alissa Sarbiewski.
Sponsored
“The first symptom was really high fever, and like, my bones ached,” Sarbiewski said.
It took her about three weeks to recover.
Then she saw a request for blood donations.
“And I was willing to do that,” she said. “I was looking for a way that I could turn the negative experience into a positive experience for somebody else.”
Sarbiewski donated to Cascade Regional Blood services, which says it’s seen a big jump in donations from people who have had Covid-19, and a lot of demand from health care providers.
Sponsored
Sarbiewski was excited to learn her blood could help other Covid-19 patients survive.
“My antibodies, my superpower antibodies that helped me fight Covid from my body can then be put into somebody suffering … and kick the Covid’s butt,” Sarbiewski said.

Dr. Seth Cohen, medical director for infection prevention at UW Medicine, said the technique has been around for over a century.
Sponsored
“It won a Nobel Prize in the early 1900s, when it was used to cure diphtheria,” Cohen said.
Cohen explained that after the blood is collected, it’s spun down and concentrated “into an enriched medium that contains these antibodies. And then you essentially just infuse it into a patient who's suffering from Covid and let somebody else's immune system try to destroy the virus.”
Cohen thinks blood plasma may offer the most hope in treating Covid-19, but cautions it’s still early. As with Remdesiver, it may take more time to know how well it really works.
So far plasma has not been approved for this use by the FDA, which says early signs are “promising” but “not yet been shown to be safe and effective as a treatment for Covid-19.” But there are several clinical trials under way, and due to this emergency, in some cases patients can receive Covid-19 plasma treatment outside those clinical trials.




