Seattle Covid-19 home testing project halted pending federal approval
The Seattle Coronavirus Assessment Network, the first of its kind nationwide, had tested more than 12,000 people to measure how prevalent Covid-19 was in the community among people with and without symptoms.
Now federal regulations have paused the project, which is run by researchers from the Seattle Flu Study and Public Health – Seattle & King County. According to the SCAN website, the project now needs a federal emergency use authorization for testing in addition to the authorization issued by the Washington State Department of Health.
“We have been in conversation with the FDA since March 1 and hope to have our EUA soon. We initiated the process to authorize our lab-developed test and self-swab kit on March 23 and, in accordance with the EUA process and timeline, submitted data to secure federal authorization on April 13. We are actively working to address their questions,” the website said.
Officials from SCAN did not immediately reply to requests for comment. A spokesperson for the FDA said that the agency does not typically discuss applications for emergency use authorizations, but that the regulations have not changed.
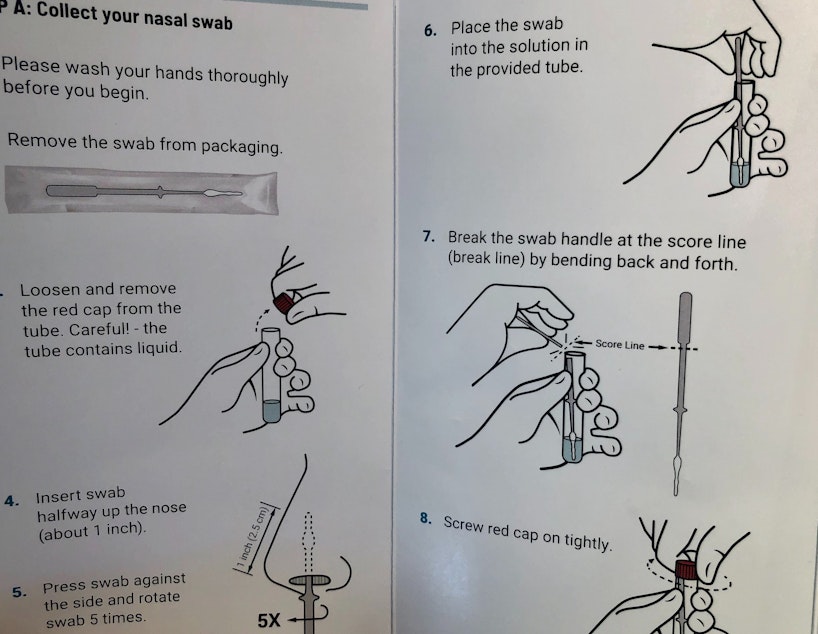
SCAN said the FDA has requested data regarding the project’s use of mid-turbinate nasal swabs, which participants swirl halfway up a nostril to collect a sample.
“Numerous scientific studies have established similar rates of detection for mid-turbinate swabs and the nasal pharyngeal swabs typically used in clinical testing, including for SARS-CoV-2 detection,” SCAN said on its website.
Sponsored
The FDA also reportedly asked about SCAN’s testing of asymptomatic people, who make up 43% of the 12,482 volunteers who have swabbed themselves or their children at home.
“This can not only help us learn more about the virus, it can help us identify positive cases of Covid-19 that might otherwise go undetected,” SCAN explained in an online FAQ.
The Seattle Flu Study home testing, has been paused, as well, pending federal approval. That study helped identify early spread of Covid-19 in the Seattle area, and also ran into federal roadblocks when the FDA stopped the lab from testing for Covid-19 early in the pandemic.
Clarifications 4:57 pm 5/14/20: The Seattle Flu Study continues, although its home testing is on hold.
Federal regulations have not recently changed, as SCAN previously stated, although a recent FDA FAQ clarified that home testing requires federal approval, not just state approval.




