CDC advisers back expansion of COVID boosters for all adults
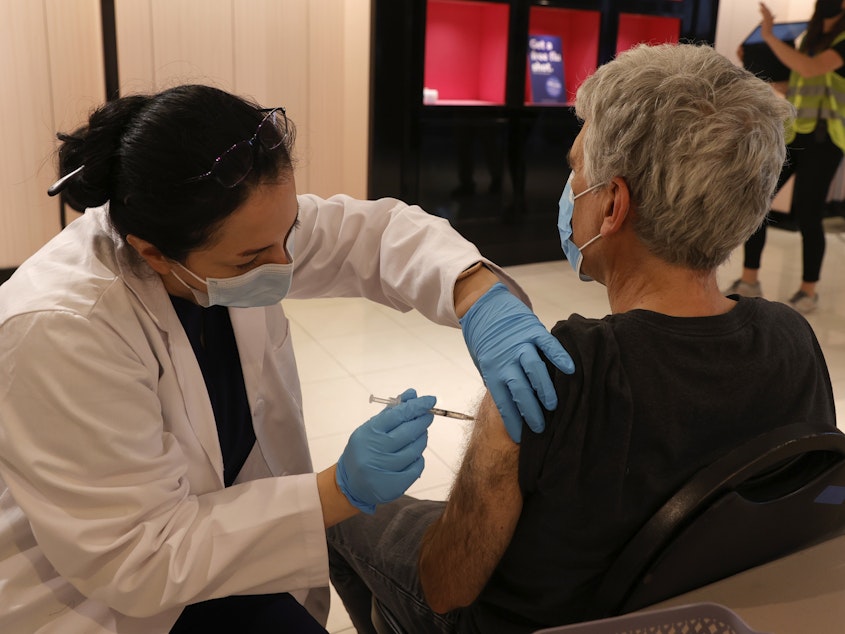
A panel of experts advising the Centers for Disease Control and Prevention backed the expansion of Moderna and Pfizer-BioNTech COVID-19 vaccine boosters to all adults.
The experts met Friday afternoon just hours after the Food and Drug Administration authorized the boosters for people 18 years and older.
The Advisory Committee on Immunization Practices voted in support of a change to COVID-19 vaccination policy that says people 50 and older should get a booster if they had a primary immunization with an mRNA vaccine (Moderna or Pfizer) at least six months before. The recommendation also applies people 18 and older in long-term care settings.
For people at least 18 and younger than 50, the panel supported a policy that says they may receive a booster based on individual risks and benefits.
An analysis from a CDC working group concluded that the balance of benefits and risks for a booster is clearest for older people. The group also noted that the latest data on myocarditis, an inflammation of the heart seen rarely after vaccination but most often in young men, is "reassuring to date."
Sponsored
The next step is for CDC Director Rochelle Walensky to issue a statement on the committee's recommendations and put forth an official position from the public health agency.
The director generally goes along with the recommendations of the panel but she overruled aspects of the committee's September decision on Pfizer's first a in a rare departure. [Copyright 2021 NPR]



