He broke his neck diving into a pool. 20 years later, new technology is helping him recover

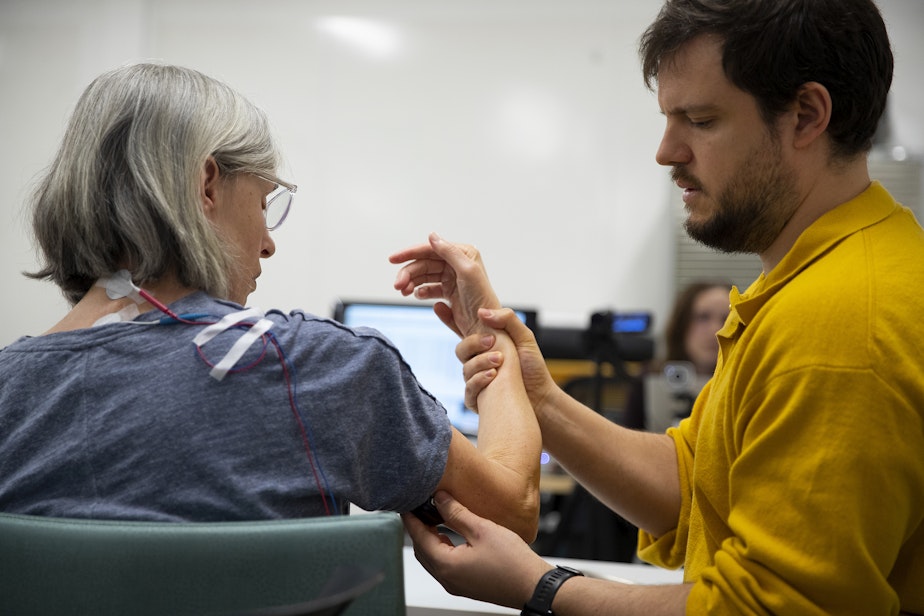
On a recent appointment, Debbie Strom sat in a chair at the University of Washington’s Amplifying Movement & Performance (AMP) Lab. She had a series of electrodes and wired sensors attached to the base of her neck, and next to her, Adrià Robert-Gonzalez, an occupational therapist, slowly ramped up the voltage pulsing through the circular nodes.
“She's just feeling a vibration right now,” Robert-Gonzalez said. “What we're trying to do is, basically, stimulate the circuitry in the spinal cord. So we're not directly stimulating the muscles, but the nervous system itself.”
Strom was taking part in new clinical trials for a treatment that delivers "transcutaneous spinal stimulation therapy" to help people with some degree of paralysis from spinal cord injury.
As electrical stimulation was applied, participants practiced a series of physical therapy exercises, ranging from pulling toothpicks from playdough to building a Jenga tower. Robert-Gonzalez closely monitored Strom as she removed and slotted white pegs into a plastic board.
“I'm watching for the movement of her fingers, right? And trying to see where she struggles, or what's more difficult,” Robert-Gonzalez said.
In a study published Monday in the journal Nature Medicine, researchers at the University of Washington, Emory University School of Medicine in Georgia, and Craig Hospital in Colorado showed that stimulation from a device designed to apply electrical current on the surface of the skin, over the spine — combined with physical therapy — improved the strength, mobility, sensation and function of arms and hands for the majority of participants.
Participants who exhibited a positive response could generally move their hands and arms at a greater range than when they started and experienced more feeling in their hands and arms. They also maintained a lot of that progress after the spinal stimulation device was turned off.
Study participants all had some level of paralysis due to spinal cord injuries that happened more than a year ago — past the window of time when recovery progress tends to plateau.
“Very few people, less than 10%, continue to recover function after that first year,” said Chet Moritz, a professor of Electrical and Computer Engineering and Rehabilitation Medicine at the University of Washington and the lead author of the study.
“That's where the spinal stimulator comes in to help give people that boost. And that additional recovery, that additional therapy that can actually improve their function,” Moritz said.

Spinal stimulation therapy devices work by providing an electrical current that activates sensory nerves as they enter the spinal cord. Those nerves go on to make synapses with motor neurons.
“What we think is happening is the sensory nerves are helping the motor neurons become ready to fire, so that any remaining signal that can make it through the spinal cord injury from the brain is able to have that immediate effect and cause that movement,” Moritz said.
Examples of movement that became easier when the stimulator was running included moving fingers to aid in eating or buttoning a shirt.
“The goal is to bring the neurons in the spinal cord closer to threshold so that your brain can turn them on when you want to move,” Moritz said.
Over the long term, the device aids in neural plasticity, which enables learning and memory. Neurons that fire together eventually wire together.
“If two neurons are active at about the same time, and one neuron causes another neuron to become active, the synapses between those neurons become stronger,” Moritz said.
Moritz and his colleagues were excited that the gains patients made often continued even after the device was detached.
“We find when we turn the stimulator off, almost all the benefits they gained during stimulation persist — and not just for a day or two, but for up to three months, as long as we've been able to measure,” Moritz said.
Moritz explained that while results varied, there were some impressive examples of improvements.
“[People] who couldn't move their fingers to even pick up a ping pong ball …immediately gaining the ability to pick up that ping pong ball,” he said. “A day later, a wooden bead or a marble.”

Study participants practiced with the stimulator, in combination with physical therapy, for an hour a day, three days a week, for a two-month period. Of 60 participants who finished the study, 43 (72%) experienced significant improvement in the strength and function of their hand and/or arm compared with gains from physical therapy alone.
Therapeutic spinal cord stimulation has already been shown to improve strength, function and range of movement in the limbs of patients with stroke and spinal cord injury. But prior treatments required people to undergo surgery for implanted electrodes that send electrical current to specific parts of the spine.
The new research shows the promise of this less-invasive therapy — electrodes on the surface of the skin — to make a lasting difference for patients.
From "the shocker" to the guitar
For people suffering from a reduced range of movement or sensation, small gains can feel like big wins. One study participant, Jon Schlueter, broke his neck diving into a pool 20 years ago, and lost control of most of his body’s function from the chest down.
“It was almost a death, really, of my body,” Schlueter said. “Mentally, physically, it was a huge change in my life, for sure. It was like starting all over again.”
Schlueter said that after 15 years of physical therapies, he’d hit a wall. That’s when his physicians at the University of Washington recommended him for an early stage of trials using an ARC-EX spine stimulator.
“As soon as that stimulator was turned on, I had instant function,” Schlueter said. “I was able to pinch a little harder and do a little bit more.”

Since using a spinal stimulation device — which he calls “the shocker” — Schlueter been able to achieve functional gains that have allowed him to indulge more fully in two of his biggest passions: cultivating his bamboo nursery, and playing guitar. At the AMP lab, he demonstrated that progress on one of his collected guitars, playing two and three-string chords as well as six-string power chords up and down the full neck of the guitar.
Schlueter says he’s also grateful that he’s been able to maintain those functional gains after the machine is turned off.
“If there's another younger person, anybody who has had a spinal cord injury — there's so much on your mind, what your life's going to be like,” he said. “I thought mine was over … and it's not, 20 years later.”
“Just keep yourself in shape for things to happen. Things are happening.”
Onward Medical — the company that developed the ARC-EX device used in the clinical trials for the Nature Medicine study — will submit data from the trials to the Food and Drug Administration in an effort to get the device approved for clinical settings.
The new treatment is relatively low-tech and easy to adopt compared with more complex neural engineering advancements that require delicate surgery and bulky equipment.
Swiss scientists, for example, have pioneered the use of a digital brain implant that uses an algorithm to interpret brain signals and communicate with spinal cord stimulators, allowing a paralyzed person to walk for short periods.
Chet Moritz said he hopes spinal stimulation therapy devices that sit on the skin will eventually be widely available to patients in physical therapists’ and occupational therapists’ offices.
Ongoing studies are also looking at how the stimulation can improve leg function for walking. Moritz says other quality-of-life issues, like bladder function, have also been shown to improve with the therapy.

“It's not a cure [for] spinal cord injury. Not everyone responds,” Mortiz said. Those in the 28% who did not see a benefit in the study, “generally … are the people with the more complete injuries, the more severe injuries.”
Compellingly for researchers, patients who have responded to the stimulation have continued to see progress with additional therapy, according to Moritz.
“Any studies that we've done that have followed up or looked at additional treatment have seen additional improvement with more stimulation,” Moritz said. “We're hopeful that the sky's the limit in terms of recovery.”
“The pace may slow down, there's often a rapid increase early in the stimulation treatment,” he added. “But we don't know what the limits are both for the maximum amount of recovery or also what other body functions that we might be able to pursue.”
Listen to the full Soundside segment by clicking play on the audio icon at the top of this story.





