Seattle-based clinical trial tweaks mRNA vaccines to fight Covid variants
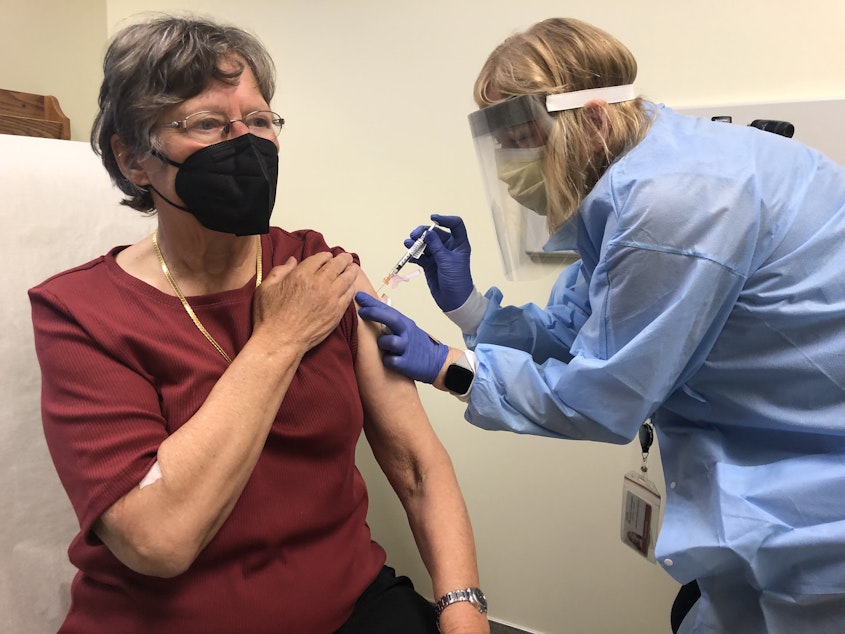
Researchers are testing a new Covid-19 vaccine and part of the clinical trial is taking place in Seattle.
The trial will tweak the existing Moderna vaccine to fight one variant of the Covid virus, B.1.351 (first discovered in South Africa).
Dr. Lisa Jackson is co-leading the study. She's a senior investigator with Kaiser Permanente’s Health Research Institute. She spoke with KUOW’s Paige Browning about the promise of this vaccine, and why this particular variant is such a huge concern.
This interview has been edited for clarity.
Dr. Jackson: The mutations increase the infectiousness of the virus. People who are infected with this variant are at least 50% more infectious to others. It also decreases the ability of antibodies and immune responses that are induced by vaccines made against the original strains of SARS-CoV-2 to work.
So, those vaccines are, now, not as effective against this variant, potentially. And lastly, it seems to cause more severe illness in people who are infected with the variant. For all those reasons, it's a great concern at this point.
Paige Browning: The vaccine that you are testing is similar to Moderna and Pfizer's. What is different about the one under trial now?
The only difference is that the mRNA component, which is the code that cells use to make proteins, is slightly different. It asks the cells to create the spike protein of the variant strain, instead of the spike protein of the original strain.
How does that work if I'm someone who were to get this vaccine? Is this a standalone shot, or is this a booster shot?
That's one of the questions. Is this vaccine good at inducing immune responses? And then, how should it be used? We're evaluating several different scenarios in the trial, one of which is to give it as a booster for people who received the original vaccine previously.
The other is to evaluate this being used in people who've never been vaccinated. They might receive the variant strain followed by the original strain, or the original followed by the variant, or even a combination of both included at the same time.
We're evaluating different doses and different numbers of vaccinations. We're trying to create a broad picture that will allow us to get an idea of how this might best be used.
There are other variants out there. Why focus on just this one?
This one appears to be a greater consequence, and you have to start somewhere, but it is true that we really can't just keep developing new vaccines for every variant that might come along, as there are new variants being detected every day.
The more genetic sequencing of the viruses that we do, the more variants we will be able to identify. Ultimately, if we had a vaccine that worked against all strains, of course that would be great.
If not, immunity induced by a variant vaccine might provide broader coverage than just to that specific variant. That will be something that's evaluated in this trial. We could perhaps get more bang for our buck out of a vaccine tailor-made to this one variant, but that potentially could act against other similar variants.
Dr. Jackson says the initial results of this clinical trial could come within a few months. The variant B.1.351 was first detected in Washington state back in January. As of April 1, there were 17 cases detected statewide.
Listen to the interview by clicking the play button above.


